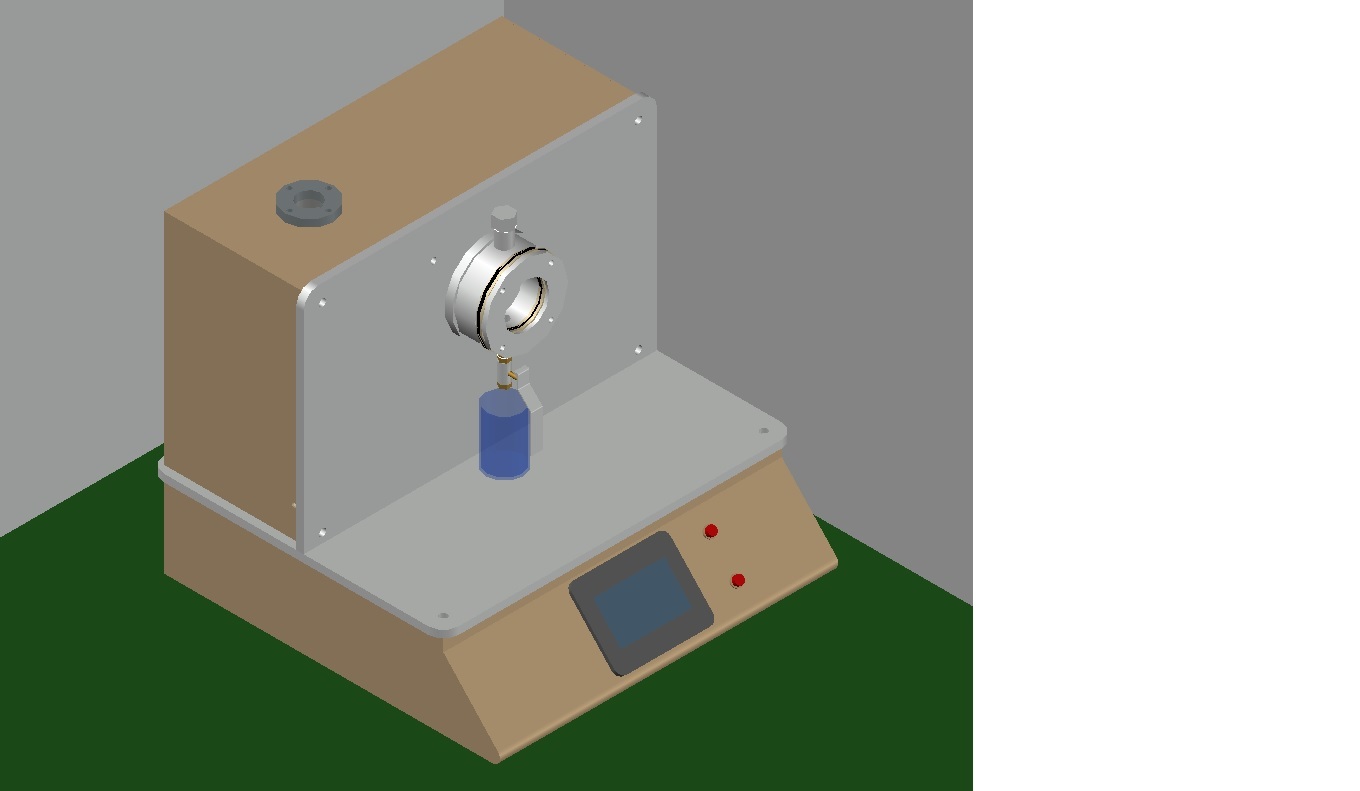
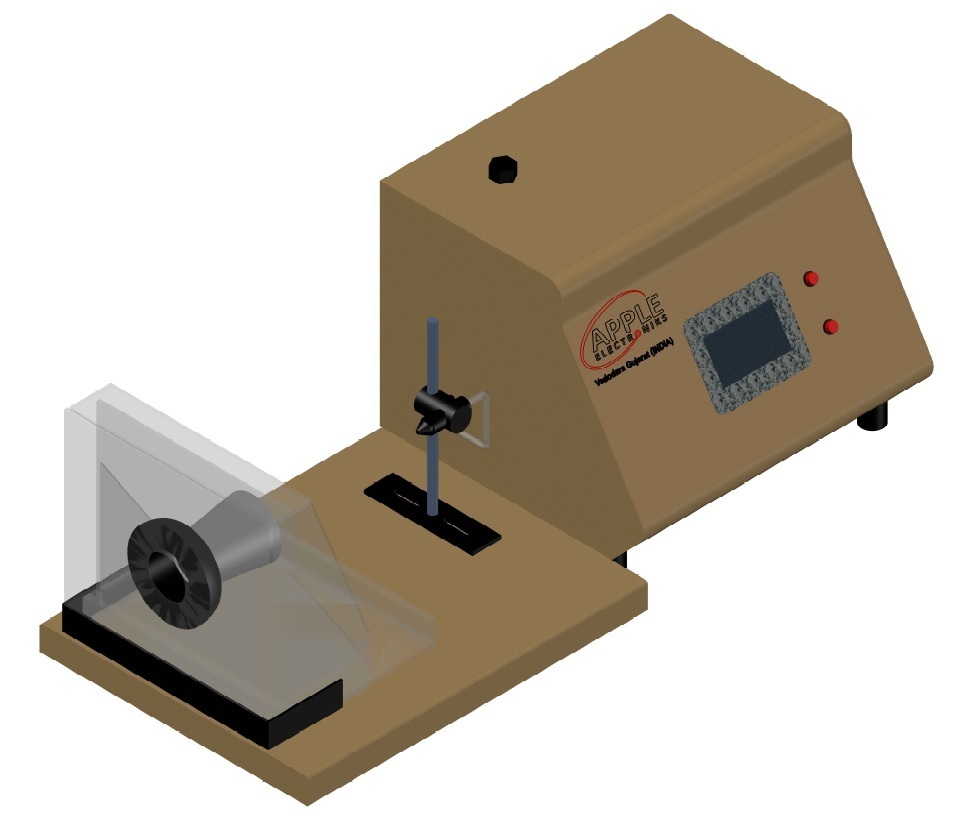
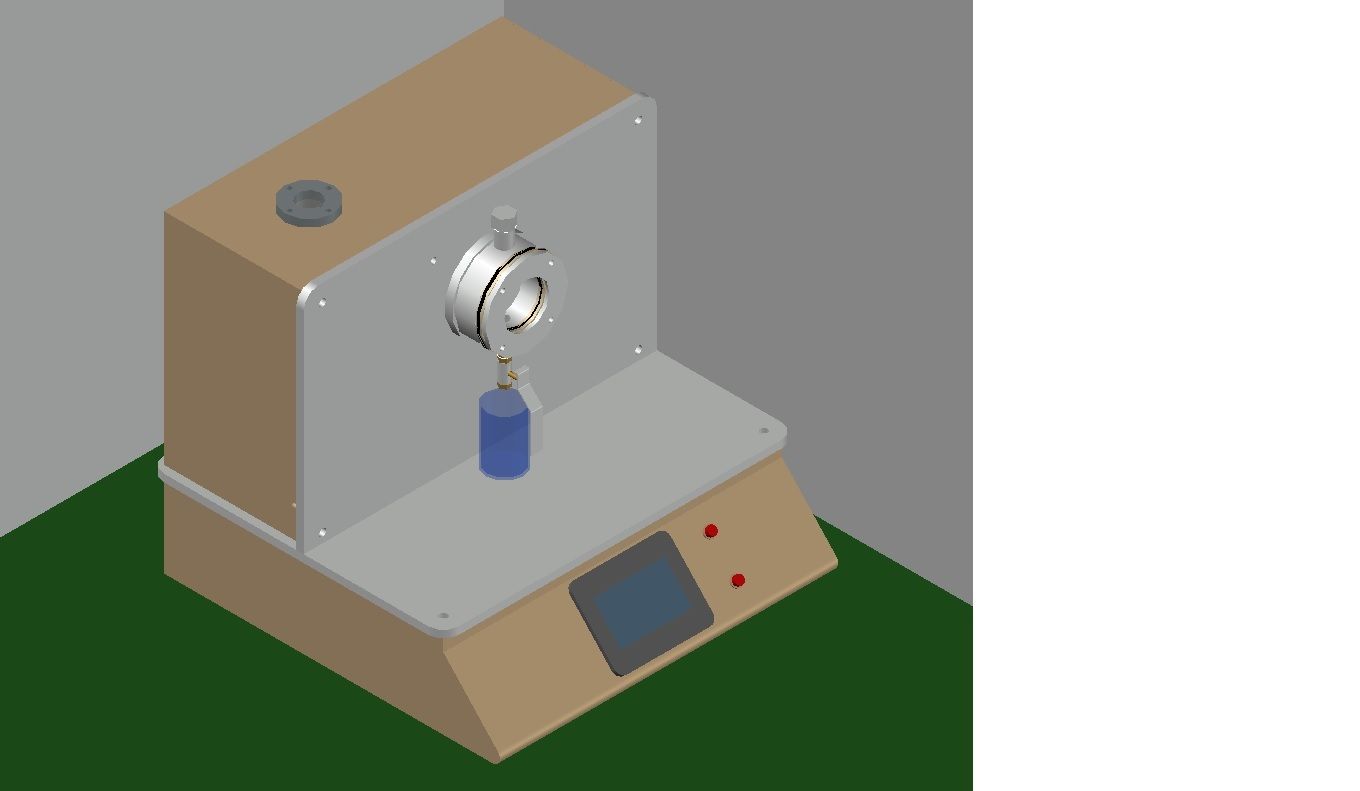
Synthetic Blood Penetration Tester
Synthetic Blood Penetration Tester Specification
- Test Range
- 0-60 kPa
- Humidity
- 50~80% RH
- Gas Pressure
- 0~30 kPa adjustable
- Number of Specimens
- Single Specimen Test with Rotating Chamber
- Specimen Size
- 80 mm x 80 mm or Customized
- Temperature
- 10C ~ 40C Operating Environment
- Test Material
- Medical Face Masks, Surgical Gowns, Protective Clothing
- Port Size
- 6 mm (Fluid Injection Port)
- Type
- Automatic, Digital Display
- Material
- Stainless Steel (Main Body), High-Quality Acrylic (Test Chamber)
- Power
- 150 W
- Power Source
- 220V AC, 50 Hz
- Dimension (L*W*H)
- 600 mm x 400 mm x 500 mm
- Weight
- 32 kg
- Usage
- Testing penetration resistance of protective apparel and medical masks against synthetic blood
- Application
- Medical PPE & Mask Testing
- Safety Features
- Automatic Door Lock, Overpressure Protection
- Control System
- Microprocessor Controlled
- Display Type
- Digital LCD Display with Touch Panel
- Compliance Standards
- Conforms to ISO 16603, ASTM F1670 Standards
- Color
- White & Grey
- Noise Level
- <55 dB
- Blood Injection Pressure Options
- 16 kPa, 21.3 kPa, 26.7 kPa
- Test Duration
- 0~99 Seconds (Adjustable)
- Accessories Included
- Specimen Holders, Cleaning Kit, User Manual
- Fluid Capacity
- 50 ml Reservoir
- Compressor Type
- Integrated, Quiet Operation
- Timer
- Digital Timer, Programmable
Synthetic Blood Penetration Tester Trade Information
- Minimum Order Quantity
- 1 Piece
- Payment Terms
- Cash in Advance (CID)
- Supply Ability
- 10 Pieces Per Day
- Delivery Time
- 60 Days
- Main Domestic Market
- , All India, South India, Central India, West India, North India, East India, Gujarat, Karnataka, Kerala, Lakshadweep, Mizoram, Meghalaya, Manipur, Andhra Pradesh, Bihar, Chandigarh, Daman and Diu, Goa, Jharkhand, Odisha, Punjab, Assam, Delhi, Dadra and Nagar Haveli, Andaman and Nicobar Islands, Arunachal Pradesh, Chhattisgarh, Haryana, Himachal Pradesh, Jammu and Kashmir, Madhya Pradesh, Maharashtra, Nagaland, Rajasthan, Sikkim, Tamil Nadu, Telangana, Tripura, Pondicherry, Uttar Pradesh, Uttarakhand, West Bengal
About Synthetic Blood Penetration Tester
Application
The medical mask synthetic blood penetration tester is used to determine the resistance of a medical respirator to synthetic blood penetration under different test pressures, and can also be used to determine the blood penetration resistance performance of other coating materials.
Features:
The ability of resistance of the medical mask to the penetration of synthetic blood under different test pressures enables that a certain amount of synthetic blood is sprayed in a horizontal direction to one side of the mask to be tested with a certain pressure and distance, and the penetrativity of the other side of the mask is observed.
The mask is fixed on the protruding fixture, and 2 ml of synthetic blood (surface tension 4.0-4.4*10E-4N/cm) is sprayed horizontally from the casing with an inner diameter of 0.84 mm to the measured mask at a distance of 300+10 mm. The injection pressure was 10.6 KPa (80 mmHg). Remove the mask and check if there is penetrating synthetic blood on the inside, which is safe and reliable.
Specifications:
- Spraying distance: 300mm-305mm adjustable
- Space between front and rear plates: 0.6cm
- Control panel: Touch-tone
- Nozzle size: 0.84mm
- Spraying speed: 450cm/s, 550cm/s, 635cm/s
- Spraying pressure: Standard/10.6kPa, 16kPa,21.3kPa/adjustable
- Nozzle length: 12.7mm
- Target plate bore diameter: 0.6cm
Standards
- ASTM F1862/F1862M-17
Power/Air: 220V 50HZ
Weight: 35 Kg
Dimensions: 1000 x 500 x 650 mm(LxWxH)
Advanced Digital Control and Safety
Featuring a digital LCD touch panel and microprocessor-controlled interface, the Synthetic Blood Penetration Tester guarantees ease of use and high-precision adjustment. Safety is prioritized through automatic door locking and overpressure protection, creating a secure testing environment without user intervention.
Versatile Testing Capabilities
With three pressure settings, programmable timer, and a rotating chamber for single specimens, this tester ensures flexibility and thorough evaluation for various protective textiles. The adjustable gas pressure and specimen size options allow for tailored assessments in line with global standards.
Designed for Modern Labs
Quiet operation (<55 dB), integrated compressor, and durable materials make this unit suitable for medical labs, PPE manufacturers, and research centers. The included accessories-specimen holders, cleaning kit, and user manual-streamline the process for efficient daily operation.
FAQ's of Synthetic Blood Penetration Tester:
Q: How does the Synthetic Blood Penetration Tester operate?
A: The tester uses a digital LCD touch panel for easy programming and selection of blood injection pressures (16, 21.3, or 26.7 kPa). A microprocessor controls test parameters and ensures consistent sample exposure in a single-specimen, rotating chamber, with the test duration adjustable from 0 to 99 seconds.Q: What materials and PPE items can be tested with this device?
A: This instrument is suitable for testing medical face masks, surgical gowns, and various protective clothing materials. The specimen size accommodated is typically 80 mm x 80 mm, but customization is possible depending on user requirements.Q: When should the Synthetic Blood Penetration Tester be used?
A: The tester is ideal for both routine quality assurance during manufacturing and research development phases when verifying a product's resistance against synthetic blood penetration is necessary, particularly for compliance with ISO 16603 and ASTM F1670 standards.Q: Where is the Synthetic Blood Penetration Tester commonly applied?
A: It is utilized in medical-equipment laboratories, quality control departments, PPE and mask manufacturing units, as well as research institutions involved in protective apparel certification and development.Q: What is the testing process for a medical mask or garment?
A: To perform a test, place the specimen in the holder and select the desired blood injection pressure and test duration on the touch panel. The chamber's automatic locking and quiet integrated compressor ensure secure and accurate results. Once the process completes, results and any observed penetration can be reviewed.Q: How does this equipment benefit manufacturers and exporters?
A: The tester enhances reliability in product development by confirming PPE and masks meet global blood penetration resistance standards. Its programmable features, digital controls, and compliance increase testing efficiency and support international market access.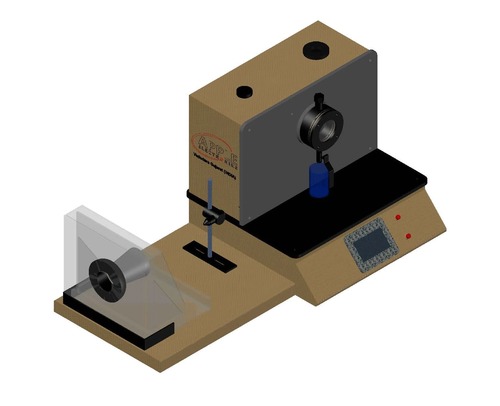

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Geo Textile Non Woven Testing Equipment Category
Liquid Strike Through Time & Wetback Tester
Price 6.95 INR / Unit
Minimum Order Quantity : 1 Unit
Usage : Industrial Use
Type : Wetback Tester
Material : SS
Weight : 45 Kg Kilograms (kg)
Cone Drop Tester
Minimum Order Quantity : 1 Unit
Usage : Industrial Use
Type : Drop Tester
Material : SS
Weight : 55 Kg Kilograms (kg)
Dry Sieving Opening Size Tester
Minimum Order Quantity : 1 Unit
Usage : Industrial Use
Type : Opening Size Tester
Material : SS
Weight : 130 Kg Kilograms (kg)
Wet Sieving Thickness Tester
Price 1700000 INR / Unit
Minimum Order Quantity : 1 Unit
Usage : Industrial Use
Type : Thickness Tester
Material : SS
Weight : 40 Kg Kilograms (kg)


 Send Inquiry
Send Inquiry






